Cutting-edge technologies are revealing the intricacies of human aging and sparking research into drugs to slow it, or even reverse it.
(Part One)
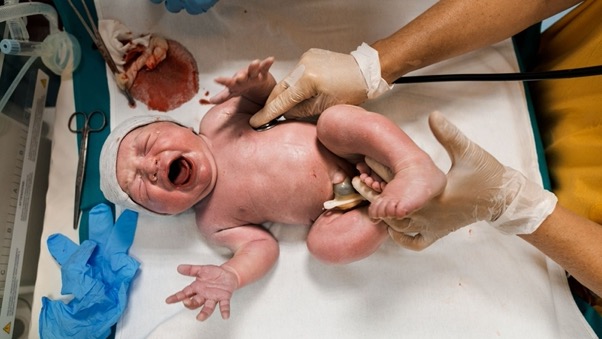
Seconds after his birth, Tommaso Citti has his vital signs checked at Beauregard Hospital in Aosta, Italy. Children born today in prosperous countries are very likely to live into their 90s. As the world grows significantly older, research into slowing or reversing aging.
Scientists are great at making mice live longer.
Rapamycin, widely prescribed to prevent organ rejection after a transplant, increases the life expectancy of middle-age mice by as much as 60 percent. Drugs called senolytics help geriatric mice stay sprightly long after their peers have died. The diabetes drugs metformin and acarbose, extreme calorie restriction, and, by one biotech investor’s count, about 90 other interventions keep mice skittering around lab cages well past their usual expiration date. The newest scheme is to hack the aging process itself by reprogramming old cells to a younger state.
“If you’re a mouse, you’re a lucky creature because there are a lot of ways to extend your life span,” says Cynthia Kenyon, a molecular biologist whose breakthrough work decades ago catalyzed what is now a research frenzy. “And long-lived mice seem very happy.”
What about us? How far can scientists stretch our life span? And how far should they go? Between 1900 and 2020, human life expectancy more than doubled, to 73.4 years. But that remarkable gain has come at a cost: a staggering rise in chronic and degenerative illnesses. Aging remains the biggest risk factor for cancer, heart disease, Alzheimer’s, type 2 diabetes, arthritis, lung disease, and just about every other major illness. It’s hard to imagine anyone wants to live much longer if it means more years of debility and dependence.

But if those mouse experiments lead to drugs that clean up the molecular and biochemical wreckage at the root of so many health problems in old age, or to therapies that slow—or, better yet, prevent—that messy build-up, then many more of us would reach our mid-80s or 90s without the aches and ailments that can make those years a mixed blessing. And more might reach what is believed to be the natural maximum human life span, 120 to 125 years. Few people get anywhere close. In industrialized nations, about one in 6,000 reaches the century mark and one in five million makes it past 110. The record holder, Jeanne Calment in France, died in 1997 at 122 years, 164 days.
Human biology, it seems, can be optimized for greater longevity. Unimaginable riches await whoever cracks the code. No wonder investors are pouring billions into trying. Google led the spending spree with the 2013 launch of Calico Life Sciences, where Kenyon is the vice president of aging research. Over the past few years, investment in the industry has come from tech tycoons, overnight crypto millionaires, and most recently Saudi royals. It seems everyone with cash to burn is placing a bet on aging’s next—or really, its first—big thing.
This work is powered by artificial intelligence, big data, cellular reprogramming, and an increasingly exquisite understanding of the zillions of molecules that keep our bodies humming. Some researchers even talk about “curing” aging.

Humans have chased dreams of eternal youth for centuries. But the study of aging and longevity was such a scientific backwater as recently as 30 years ago that Cynthia Kenyon had trouble recruiting young researchers to assist her in the experiments that would break the field open. Working then at the University of California, San Francisco, she altered one gene in tiny roundworms known as C. elegans and doubled their life span. The mutants acted younger, too, slithering friskily under the microscope while their unaltered peers lay about like lumps.
Kenyon’s startling discovery showed that aging was malleable—controlled by genes, cellular pathways, and biochemical signals. “The whole thing shifted from being out there in the nebulous world to familiar science that everyone understood,” she says. “And everyone could do it. So people just moved in.”
Delaying death in worms and mice, however, doesn’t mean it will work in humans. For a hot minute, senolytics, which kill damaging cells that accumulate with age, appeared poised to become the first antiaging therapy to make it through the regulatory gauntlet. But one of the first clinical trials, a highly anticipated study of an osteoarthritis treatment, found that it didn’t reduce swelling or joint pain any better than a placebo. Researchers and biotech companies are now testing senolytics to treat early onset Alzheimer’s, long COVID, chronic kidney disease, frailty in cancer survivors, and a complication of diabetes that can cause blindness. Clinical trials of other antiaging compounds are also under way. But so far, none of the experimental drugs that have had such dazzling effects in mice have made it to the market.
“There are lots of different approaches,” Kenyon says. “We don’t know if any of them will work. But maybe they’ll all work! Maybe combinations will be fabulous. The good news now is that people have literally accepted this kind of science as being real. They’re excited about the possibilities. We just have to try a lot of things. And that’s what people are doing.”
Walt Crompton, a retired biomedical engineer in Silicon Valley, is 69 years old. He has ample white hair, a white goatee, and a dark vision of growing older. “I’m at the age where I’m swirling around faster and faster at the bottom of the toilet,” he says. “You look around, more and more of your peers are dying, getting horrible diseases. You have little aches and pains, all of a sudden, your knee hurts when you run, and blah, blah, blah. If it’s not one thing, it’s another.”
With a mindset like that, it’s no surprise Crompton became obsessed with aging and life-extension research. He read the mouse studies. He helped out at a longevity lab. He attended conferences where scientists spoke of the “hallmarks” of aging, the interconnected ways that biology goes awry over time.

Protective caps on chromosomes, called telomeres, shorten. The genome becomes unstable and cancer-causing DNA mutations increase. Changes occur in the epigenome—compounds that latch onto DNA and regulate the activity of genes. Some cells become senescent, meaning they stop functioning normally, but like zombies, they don’t die, and they secrete chemicals that cause inflammation. Disruptions occur in pathways that respond to nutrients, lipids, and cholesterol, throwing metabolism out of whack. And the list goes on. There’s no consensus on how these changes influence one another, or which is the most important to address.
At a conference, Crompton heard a scientist named Gregory Fahy explain his theory that immunological aging could be reversed by treating the thymus, a small gland in the chest that stimulates the development of disease-fighting T cells. Fahy was seeking volunteers to test his idea that injections of recombinant human growth hormone, a drug used for decades to treat children with short stature, could rejuvenate the thymus and the body’s waning defences against disease. Fahy had injected himself with the stuff on and off for eight years, and with his thick dark brown hair and youthful enthusiasm, he appeared in enviable shape for a guy of Social Security age. Crompton signed up.
Fahy, the chief scientific officer of Intervene Immune, a California-based company, is well known as a cryobiologist who developed a technique to preserve kidneys by infusing them with ethylene glycol and storing them at minus 135°C (-211°F) until they can be transplanted. He created a stir by rewarming a rabbit brain in near-perfect condition, raising hopes a way will be found to allow mammalian brains, ours included, to survive cryopreservation. But Fahy has been fascinated by the thymus for decades, since he read a study by scientists who refreshed the immune systems of rats by implanting cells that make growth hormone. He believes most drugs that extend mice lives will disappoint us, because they “don’t do anything about keeping your immune system from going south.”


Recombinant human growth hormone is off patent, so repurposing it for antiaging won’t yield the financial bonanza of a new drug; it’s also associated with an elevated risk of some cancers. Fahy tried to get other scientists interested in doing a clinical trial and failed. “I took matters into my own hands and started regenerating my own thymus based on what I could glean from the rat study,” he says.
Because the drug can raise the risk of type 2 diabetes, he added two pills: metformin and dehydroepiandrosterone, or DHEA, a hormone that improves blood-sugar regulation. Both are also thought to mitigate the effects of aging, and they’re commonly used for that purpose. Metformin, which is taken for diabetes by 150 million people worldwide, may reduce the incidence of neurodegenerative diseases and cancer. U.S. researchers are planning a study to see if it prevents or delays major age-related illnesses. But some longevity scientists aren’t waiting: They pop metformin daily.
Crompton says he immediately felt the effects of Fahy’s regimen. “It seemed like I could leap tall buildings in a single bound.” He shed unwanted pounds without dieting. Another participant, Hank Pellissier, 70, tells me his hair, previously white, began growing in brown.
Tests showed that T cell production increased with the treatment, thymus fat disappeared, and kidney and prostate health improved. Most striking, the men lost an average of two and a half years of biological age, as measured by what’s known as an epigenetic clock. It uses blood to measure chemical changes to DNA that alter gene expression and mark the passage of time.
(More Next Week)








